Medicenna Therapeutics Announces Key Program Updates and 2026 Outlook
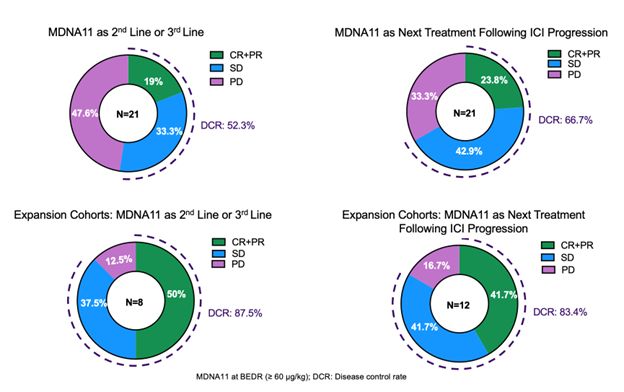
Among monotherapy expansion cohorts (n=21), ORR was 50% in patients treated with MDNA11 in 2L/3L setting and 42% when MDNA11 was the next treatment post-ICI failure, showing best-in-class potential for advanced cancer patients with no treatment options
Among all efficacy-evaluable monotherapy patients comprising 18 different cancers (n=55), ORR was19% with MDNA11 as a 2L/3L treatment and 24% when MDNA11 was the next treatment post-ICI failure
Based on these data, Medicenna plans to complete ABILITY-1 Phase 1/2 enrollment and plan registrational trial for 2L/3L melanoma and select other tumor types post-ICI failure
The NEO-CYT study, sponsored by Fondazione Melanoma Onlus, will be evaluating MDNA11 in front-line therapy for resectable advanced cutaneous melanoma with patient enrolment planned to commence in H1 2026 with interim data expected in H2 2026
MDNA113, a targeted and conditionally activated bifunctional anti-PD1-IL2 superkine, has shown a favourable safety profile in non-human primates at the highest tested dose of 30 mg/kg, prospectively supporting human dosage comparable to anti-PD-1 therapies
Management believes that the unique design of MDNA113, comprising a best-in-class IL-2 fused to a mega-blockbuster anti-PD1, will excel other bifunctional programs in development that do not include an IL-2 with demonstrable single-agent clinical activity
IND submission and initiation of first-in-human trial for MDNA113 is expected in H2 2026
Medicenna will present multiple internal and external data sets related to bizaxofusp at the 7th Annual Glioblastoma Development Summit to be held in Boston from 17-19 February, 2026
Updated cash guidance provides runway into Q3 of calendar 2026
TORONTO and HOUSTON, Jan. 15, 2026 (GLOBE NEWSWIRE) -- Medicenna Therapeutics Corp. (“Medicenna” or the “Company”) (TSX: MDNA, OTCQX: MDNAF), a clinical-stage immunotherapy company focused on the development of Superkines targeting cancer and autoimmune diseases, is pleased to provide management’s outlook for 2026, highlighting significant advancements in its key programs.
“As we head into 2026, we are building on the foundational milestones achieved in 2025 and are poised for a potentially transformative year of growth as we aim to establish Medicenna as a pre-eminent leader in the development of best and first-in-class superkine based therapeutics”, said Fahar Merchant, PhD, President and CEO of Medicenna. “We are encouraged by the updated and expanding clinical data set for MDNA11, particularly in the 2L/3L setting and after checkpoint failure, which has unquestionably positioned it as a best-in-class IL-2 super agonist. With the planned addition of MDNA113, a first-in-class IL-13 directed and conditionally activated bifunctional anti-PD1-IL-2 superkine, into our clinical armamentarium, we are well-positioned to further leverage the promise of our IL-2 platform, block-buster anti-PD1 therapy, and our T-MASK platform to advance highly precise and safer immunotherapies to treat patients with “cold” tumors that do not respond to currently available treatment options. These accomplishments reflect our team’s hard work and dedication, and we are pleased to share them with you.”
MDNA11: Promising results in the ongoing Phase 1/2 ABILITY-1 study
To date the ABILITY-1 Trial has enrolled over 110 safety evaluable patients (of which 102 are efficacy evaluable at this time) with 29 different types of cancer in monotherapy and combination arms at doses ranging from 3 to 120 g/kg without any signs of dose limiting toxicities (DLT) in patients with up to 15 lines of prior therapy and established the biologically effective dose range (BEDR) at 60-120 g/kg.
Updated MDNA11 data demonstrate the following:
- Among monotherapy expansion cohorts (n=21), ORR reached 50% in patients treated with MDNA11 as a 2L/3L treatment and 42% when MDNA11 was the next treatment line post-ICI failure, demonstrating its best-in-class potential in earlier settings post-ICI treatment failure
- Among all efficacy-evaluable monotherapy patients enrolled to-date (n=55) comprising 18 different cancers, ORR reached 19% in patients treated with MDNA11 as a 2L/3L treatment and 24% when MDNA11 was the next treatment line post-ICI failure
- Similar trends were observed in the MDNA11 + pembrolizumab combination cohorts
Impressive Monotherapy Activity of MDNA11 Observed in 2L/3L Patients and in Patients with ICI Exposure as Last Treatment Line
- MDNA11 Monotherapy Response Data in Expansion Cohorts (N=21)
- 37.5% ORR in cutaneous melanoma (2° ICI resistance) (N=8)
- 25% ORR in MSI-H cancers (N=8)
- MDNA11 + Pembrolizumab Response Data in Combination Expansion Cohorts (N=21)
- 50% ORR in MSS endometrial cancer (2° ICI resistance) (N=4)
- 43% ORR in MSS TMB-H cancers (N=7)
- 25% ORR in MSI-H cancers (N=4)
- 17% ORR in cutaneous melanoma (1° ICI resistance )(N=6)
NEO-CYT Trial: Expanding MDNA11 into Earlier Lines of Therapy
In collaboration with the Fondazione Melanoma Onlus, the NEO-CYT Trial is a randomized, multi-centre neoadjuvant study in high-risk, resectable Stage III melanoma, evaluating MDNA11 in combination with nivolumab, with or without ipilimumab. Medicenna anticipates commencement of the NEO-CYT study in H1 2026 with interim data readouts in H2 2026.
MDNA113: Preliminary Non-Human Primate Data are Promising
MDNA113, Medicenna's first-in-class, tumor anchored and conditionally activated, PD-1 x IL-2 bifunctional superkine, is advancing through preclinical development. Medicenna is planning to commence first-in-human trial in H2 of 2026.
Non-human primate studies are currently underway, with updated data reported today demonstrating its potential to dramatically widen the therapeutic index:
- MDNA113 was well tolerated, in non-human primates with no untoward clinical findings at the highest tested doses (30 mg/kg), supporting dosing in humans at or exceeding doses currently administered with standard-of-care anti-PD-1 therapies
- Lack of C-reactive protein increase following MDNA113 dosage
- Limited peripheral T cell expansion with MDNA113 despite a 30-fold increase in dosage compared to a non-masked version of MDNA113
Based on the preliminary non-human primate and previously reported pre-clinical data, management believes MDNA113 has greater potential compared to other PD-1 x IL-2 programs in development which aim to outperform mega-blockbuster commercial anti-PD-1 therapies.
Bizaxofusp: Advancing Partnering Efforts
We are committed to advance bizaxofusp towards the next stage of development through active partnering discussions, driven by the differentiated clinical data from Phase 2b study and the significant unmet need in non-resectable recurrent GBM. We will present multiple external and internal data sets from meta-analysis and bioinformatic platforms including ex-vivo studies at the 7th annual GBM summit to be held in Boston from 17-19 February, 2026. These data together with our clinical data provide compelling rationale for further development of bizaxofusp for CNS diseases.
In 2026, Medicenna will focus on three strategic priorities to drive long-term growth:
- Maximize the monotherapy and combination potential of MDNA11 in well-defined earlier-line and neoadjuvant settings to maximize market expansion opportunities
- Advance our next-generation pipeline comprising of MDNA113 as a potential first- and best-in-class targeted, masked bifunctional anti-PD1-IL-2 superkine, and demonstrate the full value and multiple opportunities of our T-MASK and BiSKIT platforms
- Advance bizaxofusp through partnership or collaboration for recurrent GBM and other brain cancers
Key Milestones Expected for 2026:
- Complete patient enrollment in ABILITY-1 study in MDNA11 monotherapy and combination arms across prioritized indications ( cutaneous melanoma, endometrial cancer, MSI-H/dMMR and MSS/TMB-H cancers) including any new expansion cohorts (for eg, CRC and NSCLC) with a focus on 2L/3L in post–anti-PD1 settings
- Report updated clinical data from MDNA11 monotherapy and combination expansion cohorts including 2L/3L and last-line anti-PD1–treated patients enrolled within the ABILITY-1 study
- Share interim clinical data from the Phase 1/2 study of MDNA11 in neoadjuvant melanoma trial (NEO-CYT) throughout 2026
- Secure FDA guidance on first potential registrational trial of MDNA11 in at least one advancer cancer indication in 2L/3L setting post-ICI therapy, including dose selection for Project Optimus
- File an investigational new drug (IND) application for MDNA113 in H2 2026 and initiate a Phase 1/2a trial by Q4 2026
- Strengthen the balance sheet through partnership and/or financing by mid-2026 in preparation for registrational trial for MDNA11 and commence FIH trial for MDNA113
- Present new pre-clinical and clinical data on bizaxofusp and IL-4Ralpha biology in recurrent GBM in Q1, 2026
- Advance and close a strategic collaboration or partnership for bizaxofusp in 2026
- Strengthen management team and board of directors throughout 2026
About Medicenna Therapeutics
Medicenna is a clinical-stage immunotherapy company focused on developing novel, highly selective versions of IL-2, IL-4 and IL-13 Superkines and first-in-class Empowered Superkines. Medicenna’s long-acting IL-2 Superkine, MDNA11, is a next-generation IL-2 with superior affinity toward CD122 (IL-2 receptor beta) and no CD25 (IL-2 receptor alpha) binding, thereby preferentially stimulating cancer-killing effector T cells and NK cells. Medicenna’s first-in-class targeted PD-1 x IL-2 bispecific, MDNA113, is in development for solid tumors and was designed using the Company’s proprietary BiSKITs™ (Bifunctional SuperKine ImmunoTherapies) and T-MASK™ (Targeted Metalloprotease Activated SuperKine) platforms. Medicenna’s IL-4 Empowered Superkine, bizaxofusp (formerly MDNA55), has been studied in 5 clinical trials enrolling over 130 patients, including a Phase 2b trial for recurrent GBM, the most common and uniformly fatal form of brain cancer. Bizaxofusp has obtained FastTrack and Orphan Drug status from the FDA and FDA/EMA, respectively.
For more information, please visit www.medicenna.com, and follow us on X and LinkedIn.
Forward-Looking Statements
This news release may contain forward-looking statements within the meaning of applicable securities laws. Forward-looking statements include, but are not limited to, express or implied statements regarding the future operations of the Company, estimates, plans, strategic ambitions, partnership activities and opportunities, objectives, expectations, opinions, forecasts, projections, guidance, outlook or other statements that are not historical facts, such as statements on the therapeutic treatment potential and safety profile of MDNA11 (both as monotherapy and in combination with pembrolizumab) and MDNA113, additional milestones, the timing and/or release of any additional clinical updates, cash runway and financing plans, the bizaxofusp potential and partnering efforts and discussions and strategic priorities. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early-stage pre-clinical or clinical studies may not be indicative of full results or results from later stage or larger scale clinical studies and do not ensure regulatory approval. You should not place undue reliance on these statements, or the scientific data presented.
Forward-looking statements are often identified by terms such as “will”, “may”, “should”, “anticipate”, “expect”, “believe”, “seek”, “potentially” and similar expressions. and are subject to risks and uncertainties. Forward-looking statements are based on a number of assumptions believed by the Company to be reasonable at the date of this news release. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can be no assurance that such statements will prove to be accurate. These statements are subject to certain risks and uncertainties and may be based on assumptions that could cause actual results and future events to differ materially from those anticipated or implied in such statements. Important factors that could cause actual results to differ materially from the Company’s expectations include the risks detailed in the latest annual information form of the Company and in other filings made by the Company with the applicable securities regulators from time to time in Canada.
The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company. The reader is cautioned not to place undue reliance on any forward-looking information. Such information, although considered reasonable by management, may prove to be incorrect and actual results may differ materially from those anticipated or implied in forward-looking statements. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date hereof and except as required by law, we do not intend and do not assume any obligation to update or revise publicly any of the included forward-looking statements.
This news release contains hyperlinks to information that is not deemed to be incorporated by reference in this new release.
Investor and Company Contact:
Shushu Feng
Investor Relations, Medicenna Therapeutics
(416) 964-5442
ir@medicenna.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/61f7710a-fa95-4db4-aa13-cfabd5232be9

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

